As already specified in the article “Saltpetre: luck and curse in the sands of the Atacama desert”, the Pacific War ended in 1883 marks a decisive turning point for Chile which, by appropriating the precious regions of Antofagasta, Tarapacá and Arica and Parinacota, acquired the title of undisputed owner of the so-called pampa salitrera, the geographical area home to all the mineral deposits in the country.
For nearly 50 years, roughly from 1883 to 1930, the extraordinary mineral wealth of these regions ensured Chile an economic prosperity beyond belief. The motivations for this economic hegemony, which was undoubtedly based on the production and marketing of saltpeter, lie on the modality around which the new mining market revolved and on the regulations that ensured a continuous and increasing entry of money into the coffers of the Chilean state. In fact, during the 50 years of Peru-Bolivian rule, more precisely from 1830 to 1879, the nitrate market had guided the fate of this area of the Atacama desert in a state monopoly regime based on a direct and absolutely exclusive industrial administration carried out by the governments of Peru and Bolivia themselves.
From the beginning of the Chilean rule, the authorities completely changed the regulation of the nitrate market by giving up the administration of the mining activity to private companies, which in most cases were foreign, and exclusively concerned about the fixing of export rights. Aside from the income that came from the sale or leasing of exploitable plots of land, direct sales of saltpetre and low-interest loans in times of economic crisis, the setting of export rights was the most profitable mode (97% of income), representing an extraordinary, continuous and growing income that allowed the country to cover more than half of public expenditure in 1910.
These growing revenues in the state coffers are undoubtedly connected to the rapid increase in the number of salitreras and, above all, to the progressive improvement of the saltpetre extraction and refining methods that followed one another throughout the nineteenth century which, undoubtedly, ensured a constant increase in the production of the precious mineral.
So what were the saltpeter production methods that followed one another until the nitrate market ended?
The use of saltpeter began in the age of Spanish colonization, in the distant sixteenth century, a period in which the mining exploitation for the extraction of gold, silver and copper officially began. Already at that time the precious properties of nitrates as key ingredients for the production of gunpowder were well known, properties that were undoubtedly given priority over their important use in agriculture as excellent fertilizers. From the sixteenth to the first decade of the nineteenth century, the only method used was the agricultural one. This consisted in the stratification of calcareous earth mixed with manure, ashes and rubble. To promote the action of the nitrifying bacterial flora, this material was periodically moved to increase the supply of oxygen and wetted with human and non-human urine, manure and lye. After about three years, the saltpetre recovery process could begin. The residual material, which was transferred to special vats, was repeatedly leached with water. The recovered liquid was subjected to cycles of boiling, cooling and crystallization of the salts present in it, including saltpetre, whose degree of purity increased after each cycle. The most widespread practice both at European and extra-European level was in fact that of setting up “nitriere” managed by private individuals under careful control of the authorities. These were nothing more than buildings in which, at night, the flocks were sheltered to allow the collection of manure. In many cases this practice was opposed by the agricultural world to be the main obstacle to fertilizing the fields.
It was in the first decade of the nineteenth century that the fortunes of the nitrate market changed thanks to the advent of the first saltpetre offices.
Five different methods, around which the pivot of the nitrate industry revolved, followed one another for about 100 years: the Olla del Indio system, the “de paradas” system, the Gamboni method, the Shanks system and the Guggenheim process.
First of all, let’s clarify one thing immediately: pure saltpetre is not found as such in nature, except in the damp and poorly ventilated walls of old houses where it is known as “white mold”. Its only natural form is represented by caliche.
How can caliche be defined?
Caliche, from Quechua “cachi”, salt, can be defined as a salt deposit of nitrates, a mixture of salts and other water-soluble substances, in particular sodium nitrate mixed with chlorides and sulphates. In particular in Chile, caliche is known as “raw Chilean nitro”, and is mixed with sand and other salts present on the surface less than a meter deep. Among the components of caliche we remember sodium nitrate (the most abundant element), potassium nitrate, sodium iodide, sodium chlorite, sodium sulphate, magnesium sulphate, calcium sulphate and sodium borate (borax). These salt depots distinguish the driest areas on the planet, in particular the Atacama Desert, which has always been regarded as the world’s largest nitrate reserve.

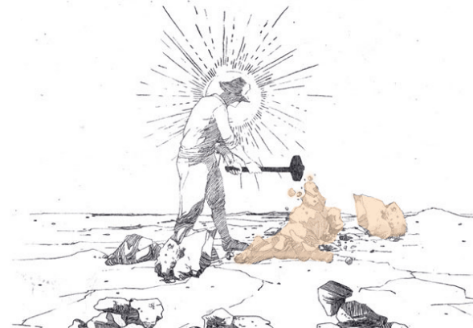
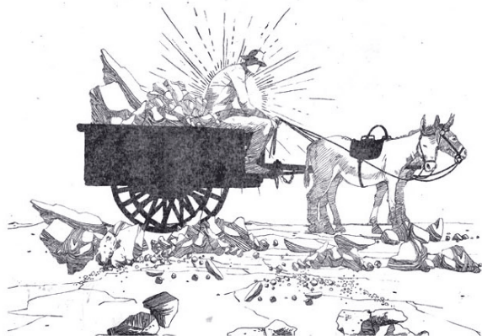
Independently on the refining method, the first phase common to all the salitreras was therefore that of extracting the caliche from the arid soil of pampa salitrera by detonation, and then proceeding to the leaching phase, which differed according to the refining system considered and which consists on the separation of the components of caliche. Originally, the extracted caliche was therefore deposited in mine wagons pushed by hand or through the exploitation of mules or horses up to large iron or copper cauldrons that were used for the leaching phase and arranged above terracotta or mud ovens in which caliche deposits, which had previously been crushed, were dissolved and heated over direct fire. The final solution was subsequently left to dry and crystallized in special trays.

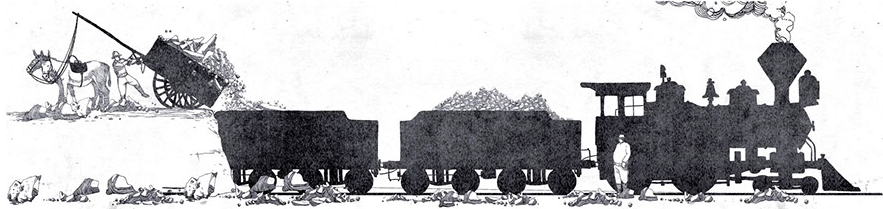
This was the procedure of the rudimentary method called “Olla del Indio” which shortly afterwards materialized in the “de paradas” system, which was the first real system of refining high quality caliche deposits (with a nitrates concentration equal to 50 or 60%). The introduction of this method led to the birth of the first salitreras (saltpetre offices) in Norte Grande (Northern Chile) and, in particular, in Tarapacá region. This process, which started being implemented from the first decade up to the 1950s and which was based on a saturation of nitrates by direct fire action, was called “de paradas” (stops system) due to the fact that once the reserves of a small mining lot were exhausted, the mining activity was automatically moved to another lot in the immediate surroundings.
Due to the total absence of machinery throughout the whole process, the “de paradas” system was undoubtedly the most depressing method for the thousands of stable workers in saltpetre offices that, right during those first decades, were beginning to outline a first rudimentary nitrate market. Considering the incessant work shifts, low wages and general sanitation conditions within the salitreras, we can only imagine the sufferings the workers had to face, this latter aspect not to be considered as a normal condition only during the first decades of the nineteenth century, but as an offstage reality that characterized the nitrate industry in Chile throughout its development.
The low performance of this process, which was mainly linked to the scarce manpower and to the total absence of machinery or even the most rudimentary industrial structure, was unable to cope with the growing demand for saltpetre, which was especially due to the progressive increase in the demand for gunpowder and to the “discovery” of its important properties as an excellent fertilizer, with a consequent increase in its use in the agricultural world starting from 1830, the year in which the exportation of saltpeter officially began. In addition to these considerations, the gradual decrease in mineral deposits rich in high quality caliche (with a nitrates concentration greater than or equal to 50%), is another important factor responsible for the need to update refining methods. The use of more efficient systems was therefore required.

1 2 3
4 5 6
“De paradas” system steps:
-
Exploration, drilling and blasting
-
Reduction, collection
-
Loading for transport
-
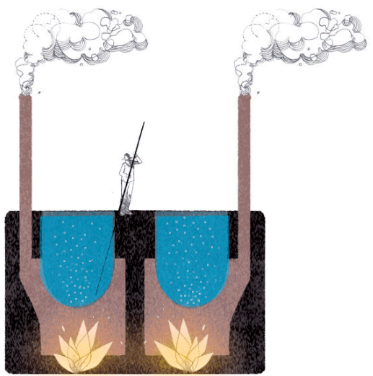
Leaching
-
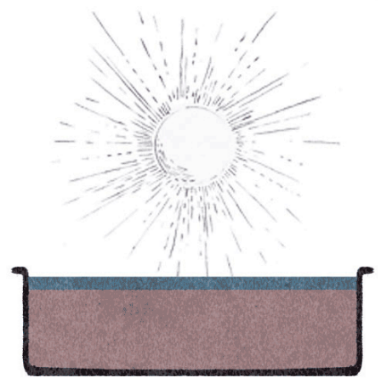
Crystallization
-
Collection of the product
It was in 1853 when a young engineer named Don Pedro Gamboni from Valparaíso introduced a system worthy of replacing the previous one and capable of meeting the increasingly demanding nature of the nitrate market. I am referring to the steam injection system or Gamboni system, the first to envisage the construction of a first elementary industrial structure in the refining process. In place of direct fire, the cutting-edge aspect of this method consisted in the production of steam in coal-fired boilers, whose direct injection into the leaching cauldrons took place through the use of a series of pipes. The use of steam allowed a more efficient dissolving of the soluble components of caliche. The solution was left to dry in the sun in special crystallization tanks. This system allowed the exploitation of quality caliche deposits of around 30%, ensuring faster and greater production of both saltpeter and iodine, the latter destined to become one of the most important complementary products commercialized.
But the real turning point and the real development of the nitrate market took place following the famous Pacific war which, in addition to the great changes in territorial and economic terms in favor of Chile, represents the real key passage from a rudimentary system to a true reality of the industrial, technical and specialized market. It was precisely from 1883-84 until the 1930s, which is known as the “Chilean period”, when the commercialization of nitrates reached its maximum expression, with a production that tripled in just ten years.
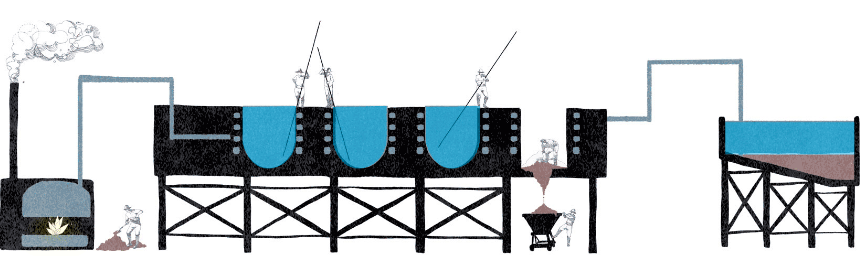
In England, a few years before the Pacific War, James Shanks had devised a system for the leaching of sodium carbonate and bicarbonate. It was Shanks’ inventiveness what stimulated the creativity of the young James Thomas Humberstone, who recognized that processing technique as a winning key for the production of saltpetre in distant Chilean lands, updating the previous system by introducing a new saltpetre dissolution method. It was 1878, the year of the inauguration of the most enduring and exploited saltpetre production system in Chile, which outlined the industrial context until the end of the long period of nitrates: the Shanks system.
This system differed from the previous one for the different method of leaching. Caliche, after being subjected to a mechanical shredding phase, was deposited in special cauldrons equipped with heating coils through which the pressurized water vapor, which was produced by coal-fired boilers, passed. This system, which ensured better leaching, allowed the exploitation of lower quality caliche deposits, even lower than 15%, avoiding the elimination of a huge amount of waste and ensuring a continuous production of iodine and borax.

Considering the numerous deposits of caliche of this quality, the diffusion of the Shanks process is directly responsible for the rapid increase of salitreras in a large part of Northern Chile and their definitive transformation into real industrial centers and fixed places of work and life for thousands of families.


1 2
3
4 5
6
Shanks system steps:
-
Exploration, drilling and blasting
-
Reduction, collection
-
Loading for transport
-
Grinding
-
Leaching and crystallization
-
Collection of the product
But it was in 1920 when things began to change. European minds never rest, especially in times of war. It was precisely the advent of the nitric acid industry in Europe what threatened the thriving Chilean market. The salitreras were all hit by news that upset the balance of nitrates market in Chile. I am talking about the synthetic saltpetre which started being produced in German laboratories through a reaction between nitric acid and potassium carbonate.
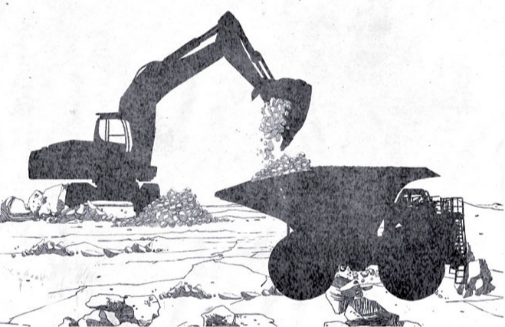
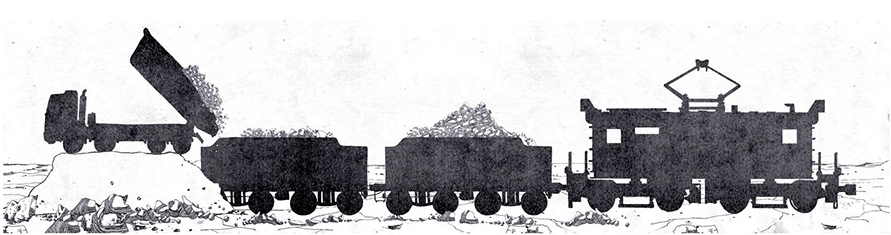
The Guggenheim system was the only method capable of keeping up with European creativity. In this case, the leaching is performed in huge vats or pools at lower temperatures than those provided by the Shanks system which were often close to the boiling point. This new system, which was almost entirely mechanized and which allowed the exploitation of low quality caliche (less than 10% of nitrates), was introduced with the aim of minimizing the consumption of calories and, in general, all production consumption. Leaching is in fact carried out at low temperatures (50 ° C); crystallization occurs by lowering the temperature to 0 ° C and stabilizers are used to eliminate impurities and by fixing sulphates, which are elements that prevent leaching at low temperatures and high yields.

1 2
3

4 5

6 7
Guggenheim system steps:
-
Exploration, drilling and blasting
-
Loading
-
Transport
-
Grinding
-
Leaching
-
Crystallization
-
Collection of the product
Unfortunately, despite the good results obtained, for almost all the nitrate offices, the necessary funds were not sufficient for a complete update of the production methods, which led almost all them to continue their activity maintaining the practice unchanged. The only salitreras who implemented this system in the region were those of María Elena and Pedro de Valdivia, who employed North American capital and technology.
Starting from 1930, one after the other the salitreras were forced to close their doors, until the total closure of all mining activities. Thus it was that the nitrate market ended forever. But other resources were waiting around the corner and which restored hope and value to Chilean purposes: copper and lithium.





0 Comments